Creating a product and getting it to market is not a one-time achievement. It entails a series of ongoing cyclical processes.
It all starts with an initial idea developed with a collaborative team. Input is gathered from all the necessary sources and then tweaks to the original concept are made as needed. Many different types of reviews, approvals, and regulatory checks are also required during this development stage. Then it’s time to provide suppliers or vendors with specific orders to ensure all the puzzle pieces will fit together as planned. Once your organization’s employees receive all the training they need to make the product according to the specifications that have been devised it feels like you’ve almost reached the finish line. Now you just flip a switch and watch as the magic is made while you celebrate your magnificent accomplishment, right?
Wrong.
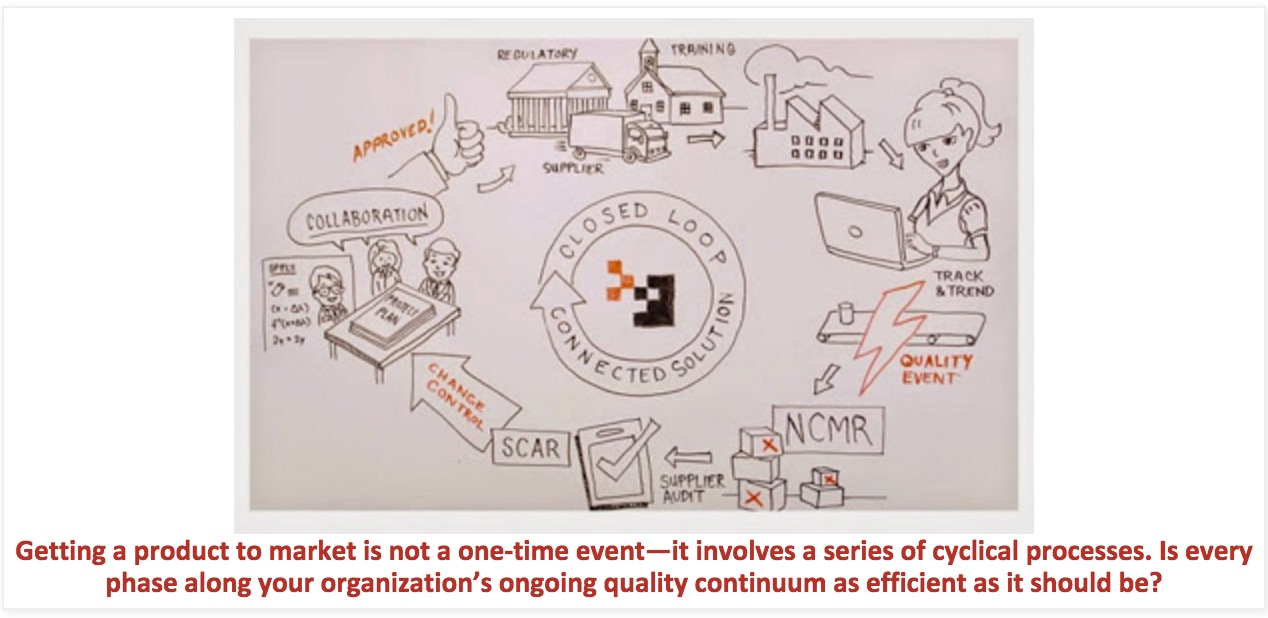 This process—collaborating, making changes, reviews, issuing new supplier specs, retraining, etc.—will reoccur again and again because quality processes are cyclical. Change is constant, so these processes have to be continually repeated. With every modification or quality event comes another recurrence of the cycle. As new factors are introduced, quality processes must be replicated with slight variations to accommodate these changes. And it doesn’t take too many repetitions of the cycle to find out whether or not your quality management system is up to snuff.
This process—collaborating, making changes, reviews, issuing new supplier specs, retraining, etc.—will reoccur again and again because quality processes are cyclical. Change is constant, so these processes have to be continually repeated. With every modification or quality event comes another recurrence of the cycle. As new factors are introduced, quality processes must be replicated with slight variations to accommodate these changes. And it doesn’t take too many repetitions of the cycle to find out whether or not your quality management system is up to snuff.
Quality cycles spiral out of control quickly if they aren’t systematic and well-organized. That’s why leading companies pay close attention to quality—they know good quality practices will enable them to avoid missteps and maintain regulatory compliance. Plus, regulatory agencies like the FDA pay close attention to quality issues since product quality can have major ramifications on the safety, satisfaction and well-being of consumers. Yet, while all of those factors come in to play, the bottom line is that excellence in quality management allows companies to get a product to market much faster so they are able to start making more money sooner.
But, you may be thinking, is it really that big of a deal to fudge quality just a bit? Surely a few minor manufacturing delays never killed anybody. And you can handle a little bit of rework here and there. Or maybe a recalled product or two. That couldn’t permanently damage your company’s reputation, could it? You could always wait and see how long you can ignore your quality system inefficiencies until regulatory intervention forces you to shut down production entirely and you have to explain to your employees why they won’t be getting their paychecks and…
You get the point.
Effective quality management is essential to getting your product out the door and onto shelves. And the implementation of a reputable electronic quality management system (EQMS) like MasterControl is proven to be the best investment a company can make to efficiently manage quality. Here’s a short list of just a few of the benefits a solution like MasterControl can provide:
- Automated document control
- A secure Web-based repository for all quality-related documentation
- Connectivity between each stage of the manufacturing process
- A guaranteed “single source of truth” for working documents
- Closed-loop quality management
- Reduced risk
- Accelerated compliance
- Products on the market faster, making money sooner
When it comes to EQMS, there are two types of people in the world: those who a) already have one that meets their needs, or b) those who enjoy searching through endless piles of paper for the one document they are certain exists somewhere only to find that it still needs to get the approval signature of person who is currently out of the office which means yet another production bottleneck. This type of problem is not farfetched, and any company that doesn’t have an automated quality system has encountered similar situations.
To find out how EQMS can help you avoid quality roadblocks and to learn how MasterControl quality management solutions can help you get your products to market sooner, check out this informative video.
(Note: This article is used with permission from GxP Lifeline and has been previously published at www.mastercontrol.com
Bio:
James Jardine is a marketing communications specialist for MasterControl Inc. He has a bachelor’s degree in journalism from the University of Utah and is based in MasterControl’s headquarters in Salt Lake City, Utah.