The Failure Mode and Effect Analysis is a great tool to evaluate the potentials risks existent in every activity or process. Take a look at my article ‘FMEA in the Kitchen.’
The Failure Mode and Effect Analysis is a great tool to evaluate the potentials risks existent in every activity or process. Take a look at my article ‘FMEA in the Kitchen.’
The PFMEA – Process Failure Mode and Effect Analysis has focus into eliminate or reduce the potential issues in a new or current process. After calculating the RPN – Risk Potential Number – it is possible to decide if preventive actions need to be done. The PFMEA is done step by step, but is necessary pay attention in some points to avoid common mistakes.
PFMEA IS A TEAM ACTIVITY
The good PFMEA is done by a team with people from several areas (Production, Engineering, Quality, and so on) and everybody needs to focus in the meeting and contribute with his or her viewpoint. Every contribution is important and need to be discussed by the team. Besides the FMEA is a structured method the creativity and exchange information and knowledge is essential to complete each step. People with different background provide a rich environment to creativity. A person that never worked in a specific process or product can see things (potential risks) that a specialist no longer can see. People with open mind can make great analysis.
It is important define previously how long the meeting will be to allow people to reserve the appropriate time. Avoid interruptions during the meeting and schedule more than one if necessary (usually it will be necessary).
DON’T PUT THE PFMEA IN THE DRAWER
Like all live document, the PFMEA needs to be up to date when there is a process modification or improvement. It reflects what is happing in the shop floor and must to be up to date to help the organization in the future improvement, process control and training new employees. It is not so unusual the PFMEA be forgot after the blitz kaizen.
The reverse FMEA is an important routine to check if what is in the paper really happen in the shop floor. There are some potential failures missing? The controls defined are in place? The occurrence of the failure is higher than expected? Maybe what was planned wasn’t put in practice and every time is time to improvements.
RELATIONS AMONG THE DOCUMENTS
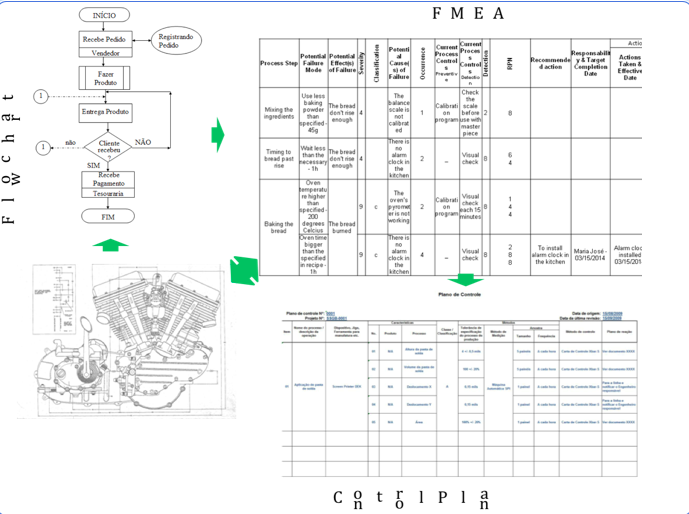
There are several links between process documentation (see Figure 1). The process sequence in the PFMEA came from the Process Flow Chart. The special characteristic in the Blue Print needs to migrate to PFMEA and Control Plan. The process controls described in the PFMEA must go to Control Plan. And there are other related documents in a process. Employees need right information at the right time. What happen when the documents don’t have the linked information? People haven’t known what is right or wrong and is not possible to guarantee perfect work.
The quality system audits check in detail if this relation was established during the product and process planning. This is one way to mitigate risks and assuring the correct information for everybody.
Figure 1 – The link between FMEA and the other documents.
THE IMPROVEMENT NEVER ENDS
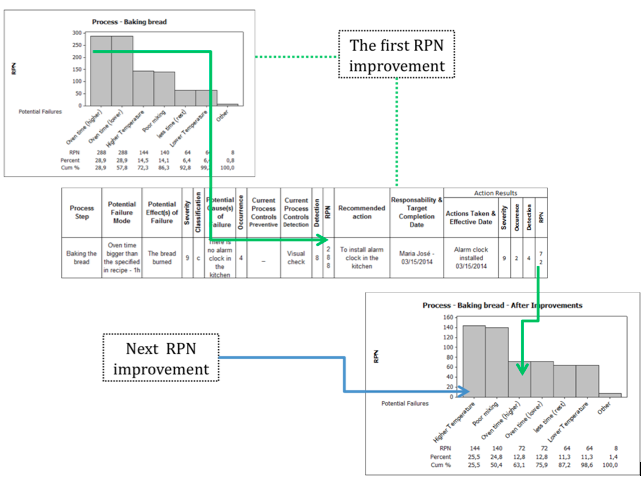
Don’t be satisfied with RPN after the first improvements. Always is possible to reduce it. Pareto’s diagram is one excellent tool to have a good view about your RPNs. We must start with actions to reduce the highest RPN and then take the new highest (see Figure 2). Of course there is investment (money) involved and is difficult to convince others to expend in things that didn’t happen. Do not lose your motivation and move yourself on with less expensive actions. But don’t forget, more than ever, do a critical analysis about these risks, their consequences and keep the boss informed. Unfortunately, living with the risk is necessary some times.
There is no doubt that FMEA is a great tool that brings good results to the organizations. And like many things in the life, the practice makes the perfection.
Bio:
Carlos E. Z. Krahembuhl has been working at autoparts companies for 20 years into Quality and Engineering areas. Master Degree in Total Quality Manager, Lean Six Sigma Black Belt and certified Quality Manager/Organizational Excellence by ASQ. Experience in Quality System (ISO/TS16949), process auditing (VDA 6.3), supplier development and problem solving.