Many companies will try to resolve compliance actions by the Food and Drug Administration (FDA) through the use of an electronic document management system (EDMS) approach that is typically post mortem.
Many companies will try to resolve compliance actions by the Food and Drug Administration (FDA) through the use of an electronic document management system (EDMS) approach that is typically post mortem.
This is risky business at two critical levels. First, no EDMS approach will replace the operation of a comprehensive quality management system (QMS) that describes the integration of necessary GxP (compliance) driven quality processes. Secondly, the response will need to have a litany of justifications for a retrospective systematic approach. This is like trying to perform process validation on pre-existing production of unqualified products: it’s bad business!
The EDMS will create frustration compounded by fallout much like rearranging deck chairs on a sinking ship. Enterprise quality software is truly a competitive approach to achieving quality. But it should only enhance the existing understanding of the dynamic approach used by a QMS. Data collection, analytics and the added review and approvals are all worked out in the maturity of a QMS, not while establishing the EDMS enterprise in response to a FDA compliance action!
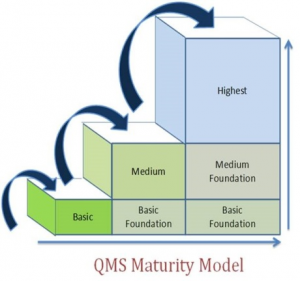
The other keys to the internal staying power of a QMS should be in improvement-based evolution of important process elements (i.e. nonconformance, customer complaints, event escalation control and preventive risk control).
In my experience performing many audits, assessments and system mapping, it can generally be said that most companies have loosely-knit quality assurance elements that aren’t necessarily working in concert like a true QMS should.
Certification of such a system does not guaranty this premise. So having some kind of quality activity occurring doesn’t qualify as a quality management system. One thing is certain; the regulators are becoming very savvy and cognizant of how a QMS should operate in order to assure quality, safety and efficacy of a product. The genesis of the quality system inspection technique (QSIT) investigation guidance and additional guidances substantiates the FDA’s recognition of the importance of developing a mature QMS.
New and insightful companion guidance documentation (i.e. GHTF, ICH) continues to point out the nature of sophistication of a QMS and how it should evolve. A handful of processes in place does not constitute a QMS. Also, a systematic approach without a sound improvement strategy doesn’t constitute a QMS either!
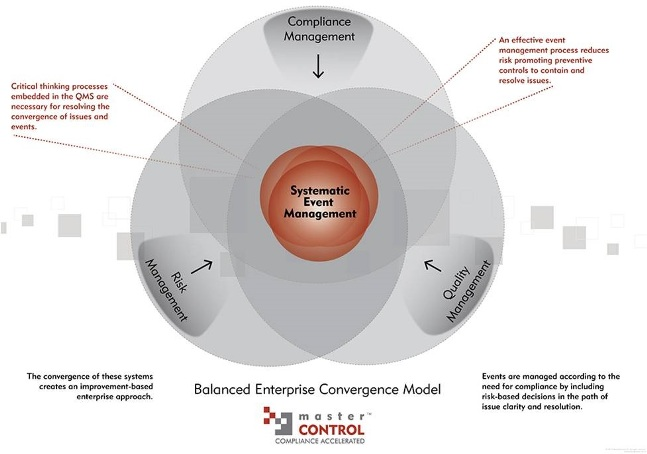
Central to any systematic approach is the convergence of how to handle and assimilate events. These events and the issues associated with them are affected by time, geography, people and ‘habits’ across several business processes. Successfully addressing these events as discrete issues requires a time-tested process for integrating compliance, risk and quality management. Only then can an enterprise EDM system truly be a cost effective enhancement to a QMS.
Bio:
Walt Murray, has performed and hosted many audits in his 30-year career. He is a veteran of the U.S. Coast Guard and has obtained certified Lead and Six Sigma Black Belt statuses during his tenure in the quality management field. He currently consults for MasterControl, based in Salt Lake City, Utah. One can learn more by accessing the website at www.mastercontrol.com